
- This event has passed.
HYP*MOL colloquium with Robert Pal
December 6, 2024 @ 1:00 pm - 2:00 pm

For the last HYP*MOL Colloquium in 2024, we will once again welcome a renowned guest. Prof. Robert Pal from Durham will tell us “A tale of two halves: Recent Advancements in Circularly Polarised Luminescence and Light Activated Molecular Nanomachines for PDT”. The event will take place on December 6 at 1 p.m. in the small lecture hall of Leipzig’s Faculty of Chemistry. Beforehand, there will again be a small snack with some coffee.
The molecular machinery of life is founded on chiral building blocks, but no experimental technique is available to distinguish or monitor chiral systems in live cell bio-imaging studies. Luminescent chiral molecules encode a unique optical fingerprint within emitted circularly polarized light (CPL) carrying information about the molecular environment, conformation, and binding state. We have built the world’s first CPL Laser Scanning Confocal Microscope (CPL-LSCM) capable of simultaneous chiroptical contrast based enantioselective live-cell diffraction limited imaging.1
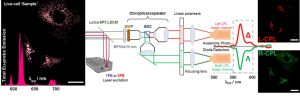
Figure 1. Simplified depiction of the CPL-LSCM developed for enantioselective differential chiral contrast (EDCC) imaging. Scale bars = 20 µm.
Parallel to thiswe have also built an all solid-state small footprint one-shot CPL photography (CPLP) camera to facilitate ad hoc time-resolved enantioselective differential chiral contrast (EDCC) imaging to enable novel intelligent Chameleon security inks to be expored.2
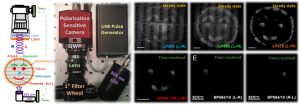
Figure 2. All solid-state camera for EDCC TR-CPLP.
(1) L. E. MacKenzie, P. Stachelek , D. Parker, and R. Pal, Nat. Commun., 2022, 13, 553- 561; L. E. MacKenzie, L.-O. Pålsson, D. Parker, A. Beeby and R. Pal, Nat. Commun., 2020, 11, 1676-1683.
(2) D. De Rosa, P. Stachelek, D. J. Black, and R. Pal, Nat. Commun., 2023, in press.; L. E. MacKenzie and R. Pal, Nat. Rev. Chem., 2021, 5, 109–124.
An important need for personalised therapeutics is the effective targeted in vivo destruction of selected cells and cell types, that are currently being highlighted by emergence of powerful optogenetic strategies. Using a new generation of light activated uni-directional molecular nanomachines (MNM, Figure 1.) we have demonstrated their application to expedite necrotic cell death using single photon excitation in the UV domain.1 and for in vivo biomedical applications using biologically safer NIR two photon (2PE) activation.2

Figure 3. Generic structure of a MNM and simplified mode of action of cell membrane penetration.
This latter direction in activation not only allow deeper tissue penetration to realise in vivo photodynamic therapy (PDT) development, but also remove UV radiation as a confounder of biomedical efficacy. Since with 2PE MNM activation will only occur in a small truly diffraction limited 3D voxel it allows the next phase of targeted photodynamic therapy protocols and methods to be designed, as with careful chemical engineering of the MNMs cell type and target receptor specific binding can be facilitated with experimental therapeutic precision as small as a single cell.3
(1) V. García-López, F. Chen, L.G. Nilewski, G. Duret, A. Aliyan, A.B. Kolomeisky, J.T. Robinson, G. Wang, R. Pal, J.M. Tour, Molecular machines open cell membranes, Nature. 548 (2017) 567–572.
(2) D. Liu, V. García-López, R.S. Gunasekera, L. Greer Nilewski, L.B. Alemany, A. Aliyan, T. Jin, G. Wang, J.M. Tour, R. Pal, Near-Infrared Light Activates Molecular Nanomachines to Drill into and Kill Cells, ACS Nano. 13 (2019) 6813–6823.
(3) J.L. Beckham, T.S. Bradford, C. Ayala-Orozco, A.L. Santos, D. Arnold, A.R. van Venrooy, V. García-López, R. Pal, J.M. Tour, Advanced Materials, 36, 2306669, 2024